- NEED HELP? CALL US NOW
- +919995411505
- [email protected]
Tarnish and Corrosion

Tarnish is a surface discolouration on a metal or even a slight loss or alteration of the surfaca finish or lustre.
Tarnish generally occurs in the oral cavity due to:
Passivation:
In certain cases the oxide film can also be protective in nature.
Eg; chromium alloys are protected from corrosion by the formation of an oxide layer on its surface which protects the metal against any further corrosion. This is known as passivation.
CORROSION:
It is not a surface discoloration but actual deterioration of a metal by reaction with the environment. Tarnush is often the forerunner of corrosion.
Water, oxygen, chloride ions, sulfides like hydrogen sulfide or ammonium sulfide contribute to corrosion attack in the oral cavity.
CLASSIFICATION OF CORROSION
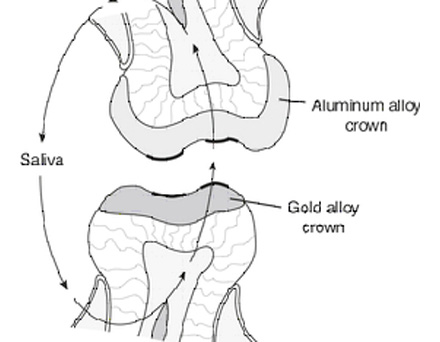
The EMF series is a classification of elements in the order of their dissolution tendencies. That is, if two metals are immersed in an electrolyte and are connected by an electricak conductor, an electric couple is formed. This metal that gives up its electrons and ionizes is calked the anode.
The metal with lowest electrode potential corrodes. Also the more active metal corrodes(anode) and the more noble metal becomes the cathode

Tarnish generally occurs in the oral cavity due to:
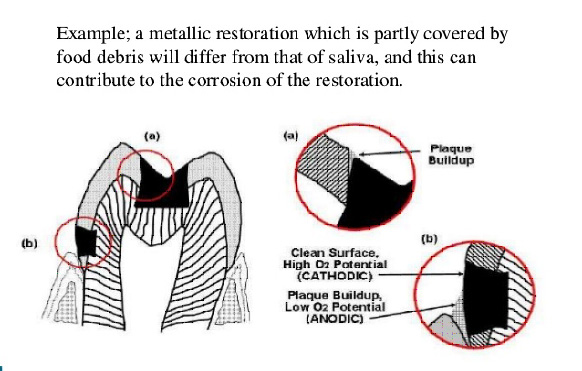
- Formation of hard and soft deposits on the surface of the restorations. Eg: calculus, mucin and plaque
- Pigment producing bacteria, produce stains
- Formation of thin films of oxides, sulfides or chlorides.
Passivation:
In certain cases the oxide film can also be protective in nature.
Eg; chromium alloys are protected from corrosion by the formation of an oxide layer on its surface which protects the metal against any further corrosion. This is known as passivation.
CORROSION:
It is not a surface discoloration but actual deterioration of a metal by reaction with the environment. Tarnush is often the forerunner of corrosion.
Water, oxygen, chloride ions, sulfides like hydrogen sulfide or ammonium sulfide contribute to corrosion attack in the oral cavity.
CLASSIFICATION OF CORROSION
| CHEMICAL/DRY CORROSION | ELECTROLYTIC/WET CORROSION |
| The metal reacts to form oxides and sulfides in the absence of electrolytes.
Eg: 1. Formation of Ag2S in dental alloys containing silver 2. Oxidation of alloy particles in dental amalgam 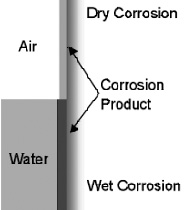 |
This requires the presence of water or other fluid electrolytes. There is formation of free electroms and the electrolyte provides the pathway for the transport of electrons.
* The anode is the surface where positive ions are formed. This metal surface corrodes since there is loss of electrons. This reaction is called oxidation reaction. * At the cathode a reaction must occur that will consume the free electrons produced at the anode and are referred to as reduction reactions. |
ELECTROMOTIVE FORCE SERIES
The EMF series is a classification of elements in the order of their dissolution tendencies. That is, if two metals are immersed in an electrolyte and are connected by an electricak conductor, an electric couple is formed. This metal that gives up its electrons and ionizes is calked the anode.
The metal with lowest electrode potential corrodes. Also the more active metal corrodes(anode) and the more noble metal becomes the cathode

TYPES OF ELECTROLYTIC CORROSION