- NEED HELP? CALL US NOW
- +919995411505
- [email protected]
Riboflavin (Vitamin B2)

Riboflavin through its coenzymes takes part in a variety of cellular oxidation– reduction reactions.
Chemistry
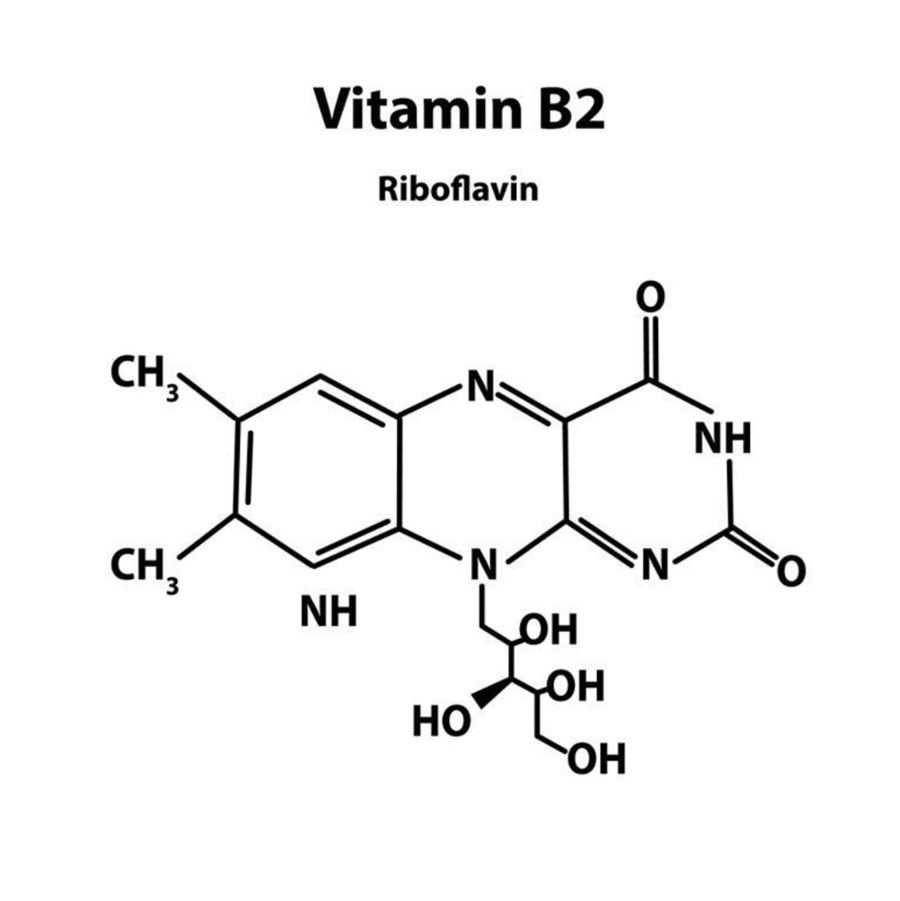
- Riboflavin contains 6,7-dimethyl isoalloxazine (a heterocyclic 3 ring structure) attached to D-ribitol by a nitrogen atom.
- Ribitol is an open chain form of sugar ribose with the aldehyde group (CHO) reduced to alcohol (CH2OH).
- Riboflavin is stable to heat but sensitive to light. When exposed to ultraviolet rays of sunlight, it is converted to lumiflavin which exhibits yellow fluorescence.
- The substances namely lactoflavin (from milk), hepatoflavin (from liver) and ovoflavin (from eggs) which were originally thought to be different are structurally identical to riboflavin.
Coenzymes of riboflavin
- Flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) are the two coenzyme forms of riboflavin.
- The ribitol (5 carbon) is linked to a phosphate in FMN. FAD is formed from FMN by the transfer of an AMP moiety from ATP.
Biochemical functions
- The flavin coenzymes (mostly FAD and to a lesser extent FMN) participate in many redox reactions responsible for energy production.
- The functional unit of both the coenzymes is isoalloxazine ring which serves as an acceptor of two hydrogen atoms (with electrons).
- FMN or FAD undergo identical reversible reactions accepting two hydrogen atoms forming FMNH2 or FADH2.
- Enzymes that use flavin coenzymes (FMN or FAD) are called flavoproteins. The coenzymes (prosthetic groups) often bind rather tightly, to the protein (apoenzyme) either by non-covalent bonds (mostly) or covalent bonds in the holoenzyme.
- Many flavoproteins contain metal atoms (iron, molybdenum etc.) which are known as metalloflavoproteins.
- The coenzymes, FAD and FMN are associated with certain enzymes involved in carbohydrate, lipid, protein and purine metabolisms, besides the electron transport chain.
Recommended dietary allowance (RDA)
The daily requirement of riboflavin for an adult is 1.2-1.7 mg. Higher intakes
(by 0.2-0.5 mg/day) are advised for pregnant and lactating women.
Dietary sources
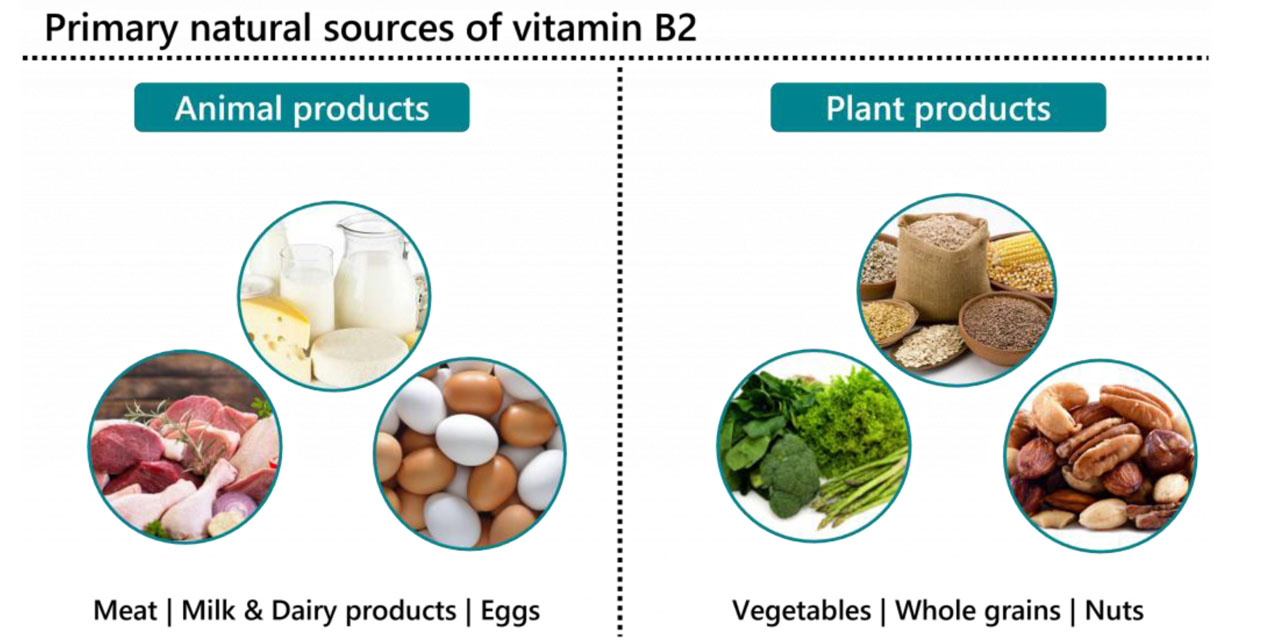
- Milk and milk products, meat, eggs, liver, kidney are rich sources. Cereals, fruits, vegetables and fish are moderate sources.
Deficiency symptoms
- Riboflavin deficiency symptoms include cheilosis (fissures at the corners of the mouth), glossitis (tongue smooth and purplish) and dermatitis.
- Riboflavin deficiency as such is uncommon. It is mostly seen along with other vitamin deficiencies.
- Chronic alcoholics are susceptible to B2 deficiency.
- Assay of the enzyme glutathione reductase in erythrocytes will be useful in assessing riboflavin deficiency.
- Antimetabolite : Galactoflavin is an antimetabolite of riboflavin.
Related posts
April 10, 2025


