- NEED HELP? CALL US NOW
- +919995411505
- [email protected]
Pharmacology Booster 4

CLINICAL TRIALS
Before a new drug comes to the market, it is extensively tested in animals and in vitro studies for safety and efficacy. If the drug is found to be promising in these studies, an application called IND(Investigational New Drug) is filed with the United States Food and Drug Administration (main regulatory authority). If the permission is granted, then drug is tested in humans. This testing is called clinical trials. These are divided into 4 phases.
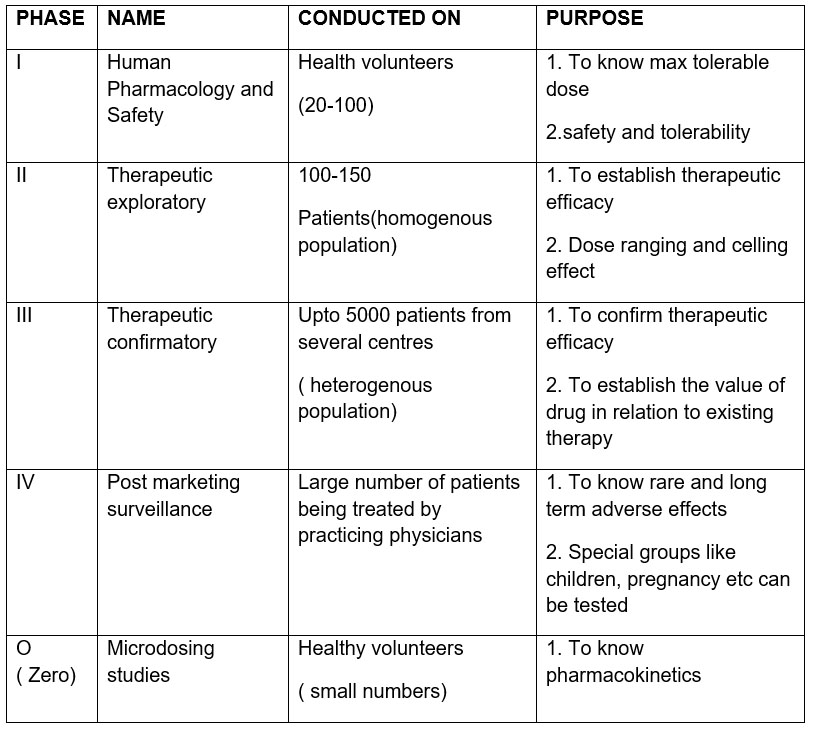
Q. Which phase of clinical trials is done after the drug enters the market ( AIIMS May 2016)
A) Phase I
B) Phase II
C) Phase III
D) Phase IV
Ans: D, Phase IV- post marketing surveillance
Related posts
April 10, 2025
April 9, 2025
April 4, 2025




