- NEED HELP? CALL US NOW
- +919995411505
- [email protected]
Causes of tooth discoloration
- Extrinsic discoloration-
extreme stains are located on the outer surface of the teeth.
- Intrinsic discoloration-
Intrinsic stains are those which are internal or present within the two structures.
- Stain internalisation
Those circumstances where extrinsic stain enters the Tooth through defects in the tooth structure.

Bleaching materials
Hydrogen peroxide
- 30 to 35 percentage stabilised aqueous solution are the most common-superoxol.
- Colourless, clear odourless liquid, Unstable should be kept away from heat, stored in lightproof amber bottles.
Carbamide peroxide
- Also known as urea hydrogen peroxide, bifunctional derivative of carbonic acid.
- Available in the concentration range of 3 to 45 percentage.
- Mean pH 5 to 6.5
Sodium perborate
- When fresh, it contains about 95 percentage perborate, corresponding to 9.9 percentage of the available oxygen.
- Sodium perborate is stable when dry.
- Three types of sodium perborate preparations are available monohydrate, trihydrate, tetrahydrate.
- They differ in oxygen content, which determines there bleaching efficacy.
Mechanism
- The bleaching process is based on the oxidation of bleaching agent.
- Redox reaction takes place in the bleaching process.
- Before the bleaching process, teeth is the reducing agent and bleaching material is the oxidising agent.
- After bleaching, tooth is oxidised i.e. Organic pigment of the tooth is oxidised and the bleaching material is reduced.
- The free radicals produced by the peroxides are per hydroxyl and nascent oxygen.
- Per hydroxyl is responsible for better bleaching action.
High molecular weight complex organic molecules Unsaturated double carbon bond + Bleaching agent =
Low molecular weight organic molecules -saturated carbon bonds.
Non vital bleaching
- Thermocatalytic or in office technique
- Walking bleach or out of the office or home technique
Vital bleaching technique
- Power bleach or in office technique
- Night guard or dentist prescribed home technique
Thermocatalytic or in office technique

It involves the placement of 35% of hydrogen peroxide liquid into the debrided pulp chamber and acceleration of the oxidation process by placement of a heating instrument into the pulp chamber.
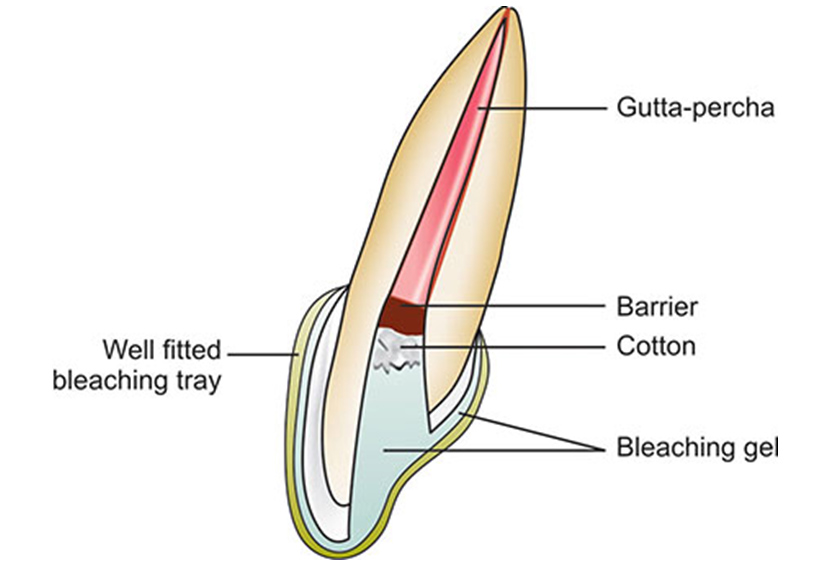
Walking bleach or home technique
In this technique either superoxol ie 30% H2O2 by volume or a mixture of sodium perborate and superoxol is used.
Power bleach or in office technique
In this technique, 35 % of H2O2 is used and the oxidation process is accelerated by applying heat or intense light.
In this technique high intensity light, which was used as a heat source is replaced with conventional halogen units, plasma arc lamps, light lights ,xenohalogen And laser
Advantages
- fast result
- avoid problems of home bleaching
Disadvantages
- expensive
- increased in office time
- dehydration of teeth resulting in false light shade
- caustic nature of 35 to 50 percentage hydrogen peroxide
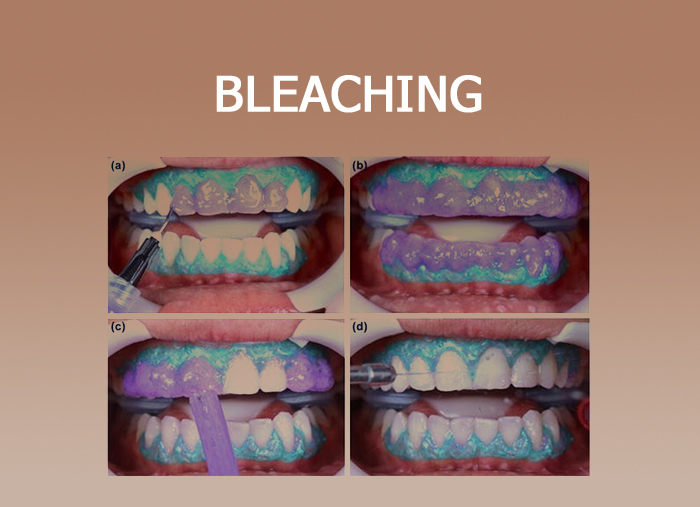
Night guard bleach or home technique
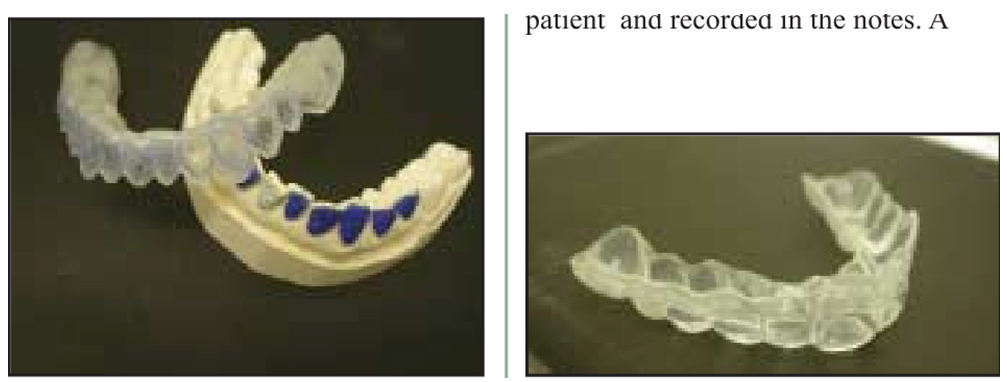
- In this technique, 10-15% of carbamide peroxide is applied with the help of bleaching trays.
- Carbamide peroxide degrades into 3% hydrogen peroxide and 7% urea.
- Night guard bleaching home bleaching technique
Advantages
- Simple and fast
- Symbol for dentist to monitor without extended clinical time
- It is cost effective
- It is not usually a painful procedure
- Patients can bleach their teeth at their convenience
- Results relatively quick
Disadvantages
- Patients need to participate activity in their treatment
- The colour changes depended on the amount of time the trays are worn.
- This system may be open to abuse by using excessive amounts of bleach for too many hours per day.
- It is difficult for patient who react easily to tolerate the bleaching trays in their mouth
Side effects of night guard
- Gingival irritation
- Soft tissue irritation
- Altered taste sensation
- Tooth thermal sensitivity
Laser bleaching technique
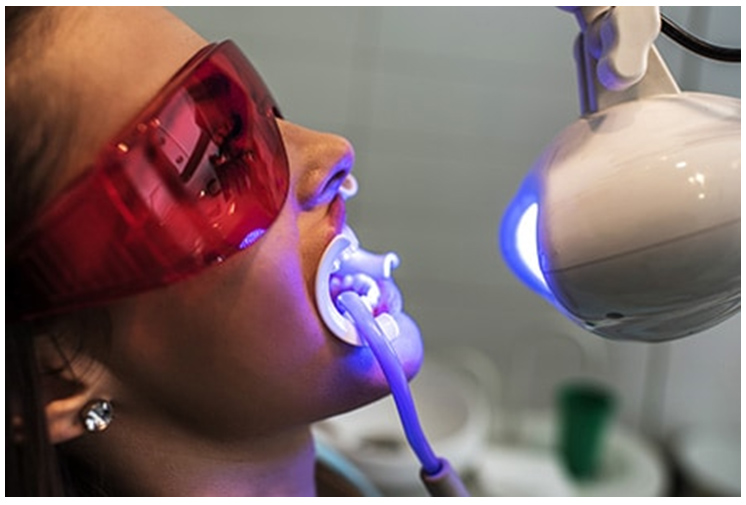
When the source of activation is laser it is known as laser bleaching technique
Carbon dioxide argon and diode are the types of lasers
Advantages of laser bleaching
- faster
- it might help to remove difficult stains caused by tetracycline and fluorosis
Disadvantages
- expensive
- post operating sensitivity can be high
- Cervical resorption is a common consequence of bleaching of non-vital teeth whereas apical periodontitis is for vital teeth.
|




