- NEED HELP? CALL US NOW
- +919995411505
- [email protected]
APOPTOSIS
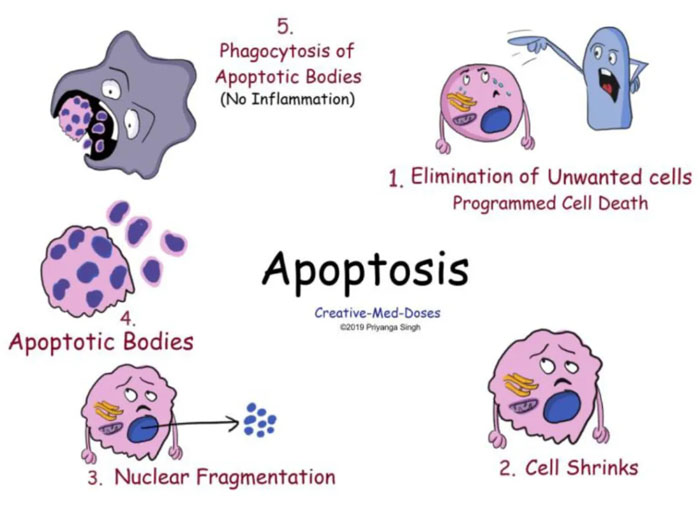
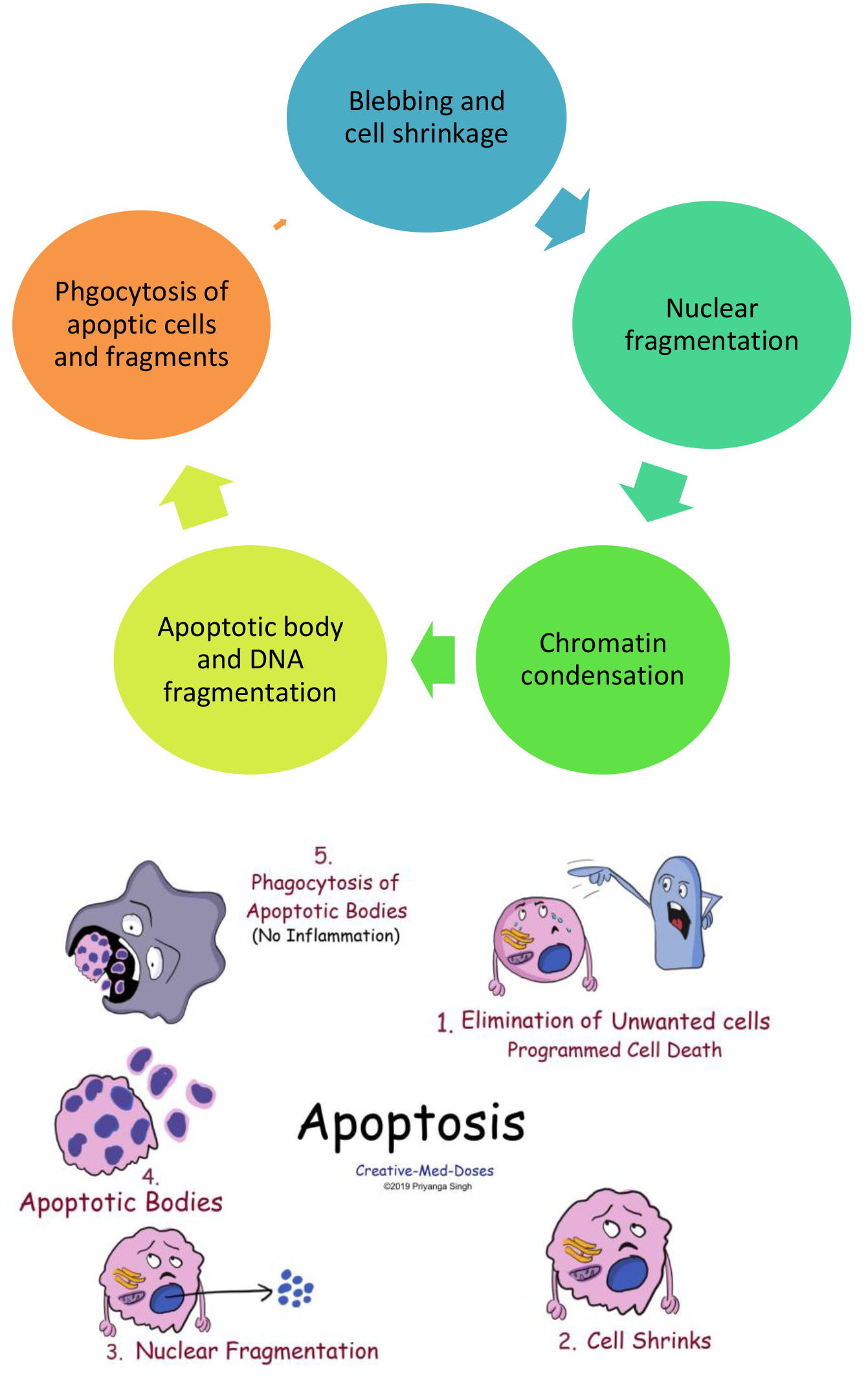
A type of cell death in which a series of molecular steps in a cell lead to its death.
Apoptosis is a Greek word that refers “dropping off” or “falling off,” as in leaves from a tree.
This is one method the body uses to get rid of unneeded or abnormal cells.
Apoptosis in physiological conditions
In human body about 100,000 cells are produced every second by mitosis and a similar number die by apoptosis
History
- German scientist Carl Vogt was first to describe the principle of apoptosis in 1842.
- In 1845, anatomist Walther Flemming delivered a more precise description of the process of programmed cell death.
- In 1972 Kerr first introduced the term apoptosis in a publication
- The 2002 Nobel Prize in Medicine was awarded to Sydney Brenner, Horvitz and John E. Sulston for their work identifying genes that control apoptosis.
- The genes were identified by studies in the nematode C. elegans and these same genes function in humans for apoptosis.
Importance of apoptosis
- Recognized and accepted as a distinctive and important mode of “programmed” cell death, which involves the genetically determined elimination of cells.
- Apoptosis is essential to embryonic development and the maintenance of homeostasis in multicellular organisms.
- In humans, for example, the rate of cell growth and cell death is balanced to maintain the weight of the body.
- During fetal development, cell death helps sculpt body shape and making the right neuronal connections.
- Tissue homeostasis mainly depends on the balance between cell proliferation and cell death.
- Programmed cell death or apoptosis is an intrinsic death program that occurs in various physiological and pathological situations .
- The process of apoptosis may be blocked in cancer cells.
Biochemical events lead to characteristic cell death.
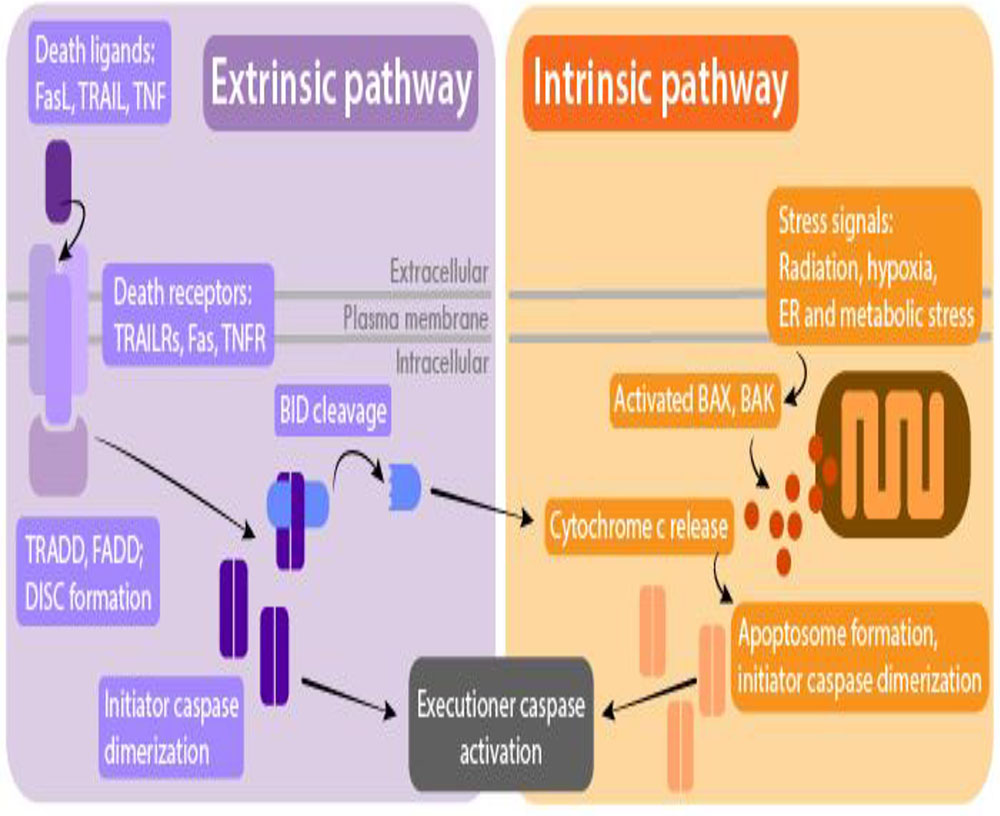
Pathways of apoptosis
Apoptosis cannot stop once it has begun, it is a highly regulated process. Apoptosis can be initiated through one of two pathways
-
Intrinsic pathway
-
Extrinsic pathway
The intrinsic pathway to apoptosis
- Triggered by stress or damage to the cell.
- In response to these damages or stresses, the cell activates a set of proteins called “BH3-only proteins.”
- BH3-only proteins are a class of proteins including several pro- and antiapoptosis proteins.
- Pro-apoptotic BH3-only proteins activate BAX and BAK
- Activated BAX and BAK cause a condition known as “MOMP.” MOMP stands for “mitochondrial outer membrane permeability.”
- MOMP is considered the “point of no return” for apoptosis.
- The steps leading up to MOMP can be stopped in their tracks by inhibitor molecules, but once MOMP has been achieved, the cell will complete the death process.
- MOMP plays its key role in apoptosis by allowing the release of cytochrome C into the cytoplasm.
- Under normal circumstances, cytochrome C plays a key role in the mitochondrial electron transport chain
- Cytochrome-C in the cell cytoplasm prompts the formation of the ominous-sounding “apoptosome” – a complex of proteins that performs the final step to beginning cellular breakdown.
- The apoptosome, once it is formed, turns pro-caspase-9 into caspase-9.
- Just as with the activation of caspases-8 and -10 in the extrinsic pathway to apoptosis, caspase-9 is able to trigger further changes throughout the cell
- Caspase-9 performs several functions to promote apoptosis. Among the most important is the activation of caspases-3 and -7.
- Once activated, caspases-3 and -7 begin the breakdown of cellular materials. Caspase-3 condenses and breaks down the cell’s DNA.
Extrinsic Pathway
- In the “extrinsic” pathway to apoptosis, a signal is received from outside the cell instructing it to commit programmed cell death.
- This may occur if the cell is no longer needed, or if it is diseased.
- Two common types of chemical messengers that trigger the extrinsic pathway to apoptosis are FAS and TRAIL. These molecules may be excreted by neighboring cells if a cell is damaged or no longer needed.
- The receptors that bind to FAS and TRAIL are called “FASR” for “FAS Receptor” or “TRAILR” for “TRAIL Receptor.”
- As with most receptor proteins, when FASR and TRAILR encounter to their signal molecule – sometimes called a “ligand” – they bind to it.
- The binding process causes changes to the receptor’s intracellular domain.
- In response to the changes in the intracellular domain of TRAILR or FASR, a protein inside the cell called FADD also changes.
- FADD’s name is either amusing or terrifying: it stands for “FASAssociated Death Domain” protein
- Once FADD has been activated by changes to the receptor, it interacts with two additional proteins, which go on to start the process of cell death.
- Pro-caspase-8 and pro-caspase-10 are inactive proteins until they interact with an activated FADD. But if two of these molecules encounter an activated FADD, the parts of the proteins that keep them inactive are “cleaved” or “cut” away.
- The pro-caspases then become caspase-8 and caspase-10 – which have been romantically referred to by scientists as “the beginning of the end” due to their role in starting apoptosis.
- Caspases-8 and -10 disperse through the cytoplasm and trigger changes to several other molecules throughout the cell, including messengers that start the breakdown of DNA after being activated by the caspases.
- Another inactive molecule called BID is transformed into tBID when the activated caspases cleave off the part of BID that keeps the molecule inactive.
- After BID is transformed into tBID, tBID moves to the mitochondria. tBID activates the molecules BAX and BAK.
Related posts
April 10, 2025
April 9, 2025
April 4, 2025




