- NEED HELP? CALL US NOW
- +919995411505
- [email protected]
1. Which one of the following statements is correct?
A. Corrosion is forerunner of tarnish
B. Tarnish is forerunner of corrosion
C. Tarnish and corrosion are not related to each other
D. Tarnish causes pitting of restoration
Ans. B
2. A highly polished surface on a metallic dental restoration aids considerably in the prevention of the
A. Dimensional change
B. Thermal conductivity
C. Warpage
D. Tarnish and corrosion
Ans. D
3. Resistance to corrosion in a cobalt chrome casting is due to presence of
A. High quality iron
B. Chromium
C. Cobalt
D. Nickel
Ans. B
4. Corrosion rate of nickel is reduced by
A. Coating nickel with paint
B. Coating nickel with zinc
C. Coating nickel with aluminium
D. Coacting nickel with chromium oxide
Ans. D
Chromium, aluminium and titanium form strong adherent oxide films on their surface to protect from corrosion. This is called passivation.
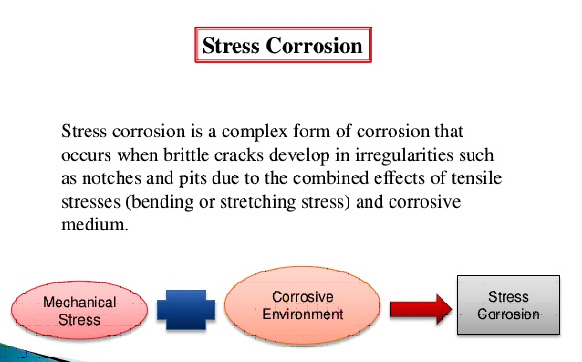
5. Corrosion occuring due to variation in the electrolytes or in the variation of composition of a given electrolyte in the system is know as
A. Galvanic corrosion
B. Crevice corrosion
C. Corrosion due to heterogenous compound
D. Stress corrosion
Ans. B
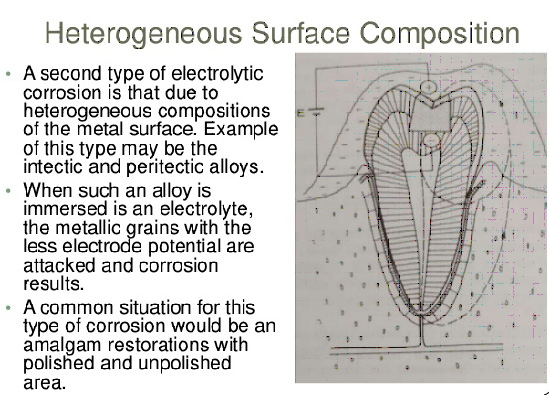
6. Galvanism is
A. Tarnishing
B. Electrolytic corrosion
C. Chemical corrosion
D. Annealing
Ans. B
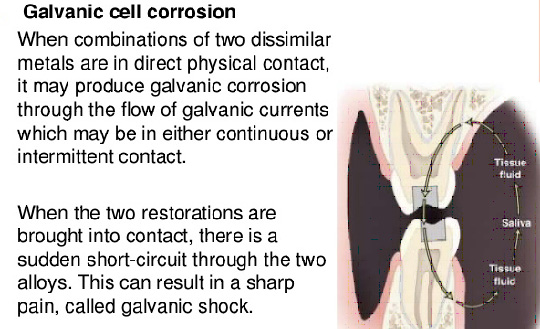
7. Galvanic shock is seen when
A. Amalgam filling approach gold crown in occlusion
B. Amalgam and gold crowns adjacent to each other
C. Amalgam and gold crowns in opposite quadrants of the same arch
D. All of the above
Ans. A
8. Which of the following metals possess greatest EMF value?
A. Gold
B. Platinum
C. Mercury
D. Silver
Ans. A
9. Gold has electrode potential value of
A. +1.36V
B. +0.86V
C. +0.80V
D. +1.5V
Ans. D
10. In the electrochemical corrosion of silver amalgam which of the following act as cathode and anode
A. Tin is anode and silver is cathode
B. Mercury is anode and silver is cathode
C. Mercury is cathode and tin is anode
D. Mercury is anode and silver is cathode
Ans. A
| METALS | ELECTRODE POTENTIAL(V) |
| MERCURY | +0.80 |
| SILVER | +0.80 |
| COPPER | +0.47 |
| TIN | -0.14 |
| ZINC | -0.76 |